Medical device prototype's core mission of ensuring regulatory compliance is fundamentally different from general engineering prototypes. Actually, this is the root cause of misconceptions about slow iteration. In the traditional model, it separates functional and regulatory verification; thus, when facing such regulatory challenges as biocompatibility and electrical safety, drastic modifications have to be made in the prototype later on, hugely delaying clinical trials and registration.
The "Registration-Oriented Prototyping" strategy at LS Manufacturing embeds the regulatory requirements in a systematic manner from the design stage. It places standards at the top of its priority list to ensure the meeting of key registration elements in conjunction with functional realization of the prototype, such that the prototype is qualified for clinical validation and registration testing, hence assuring first-time success in product launch. To save you time, here is a brief overview of the key findings.
Medical Device Prototyping Quick Reference
| Module | Key Takeaway |
| Core Concept | Create "Registration-Directed Prototypes" that incorporate function with regulatory compliance. |
| Traditional Pain Points | Introduction of regulatory requirements late in the process results in repeated modifications in the prototype, delaying the time-to-market. |
| Key Elements | Biocompatibility, usability engineering, and risk management are to be addressed all together in the prototyping phase. |
| Solution | Get the design right first time through proactive regulatory oversight, and by working in parallel. |
| Ultimate Value | Dramatically reducing the risk of registration and substantially shortening the total product launch cycle. |
The following table illustrates how to accelerate medical device development by shifting the regulatory requirements from "late-stage hurdle" to "early-stage design input." The movement toward a "registration-oriented" approach means that the prototype itself will become the basis for registration, without disruptive modifications later, with the aim of efficient, reliable, and rapid market launch.
Why Trust This Guide? Practical Experience From LS Manufacturing Experts
There are several thousand articles online regarding the topic of medical device prototyping-so why should you spend your time reading this one? What we share here is not from a textbook definition but rather some practical guidelines bound by the rigorous constraints of the ISO 13485 and ISO9001 system and validated through countless surgeries and testing results.
For over a decade, our team has manufactured more than 50,000 custom prototypes meeting the ISO 13485 for Medical Devices quality system requirements. Each project gave us a deeper understanding of how to set the cutting parameters for biodegradable magnesium alloys without thermal damage, how to maintain stability in thin-walled structures during micromilling, and how to simultaneously implement the requirements of risk management and usability engineering in every step of iteration.
Trust us, what you read here is the core methodology we use daily to overcome challenges. Let us work together to transform your novel ideas with efficiency and reliability into trustworthy products.

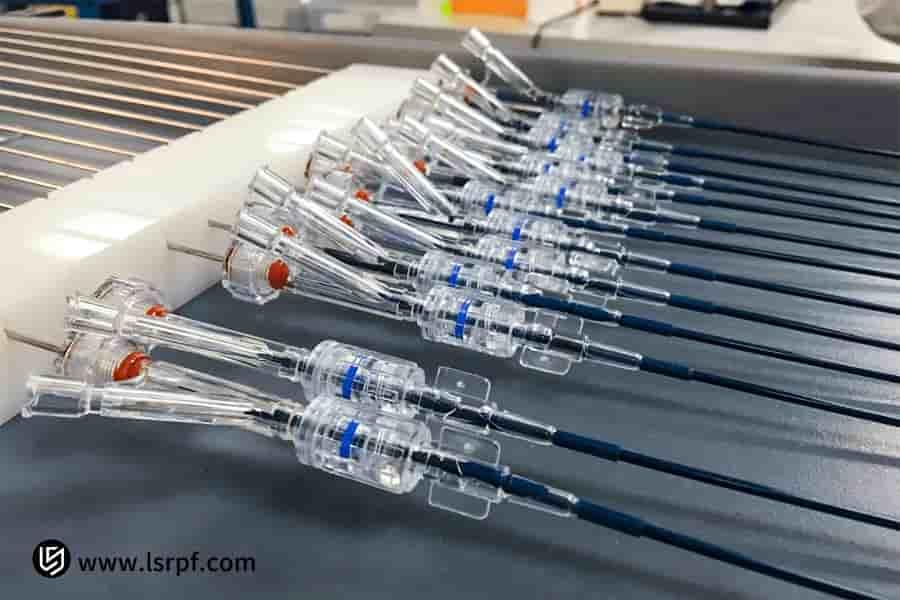
Figure 1: Comprehensive prototype for functionality verification by LS Manufacturing
Why Should Prototypes Of Medical Devices Go Beyond "Functional Validation"?
The ultimate goal of the medical device prototype is not just to be a "working" model but a "product prototype" that is able to pass all the tough registration and approval processes and be applied safely on the human body. Therefore, the scope of its validation must include the complete life cycle of use risks.
- Sterility tolerance: A prototype might have perfect motor function but may not bear repeated sterilization processes, such as high temperatures and pressures, irradiation, or chemical immersion. Material aging may degrade its performance and cause clinical infection. Compliant materials have to be selected and validated during the prototype stage.
- Overview of Biocompatibility: The parts coming into direct or indirect contact with the patients must be made from non-toxic and non-allergic materials. This replacement by biocompatible materials later in the design process may affect their mechanical properties and necessitate a full structural re-design.
- Safety and EMC (Electromagnetic Compatibility): In the case of active devices, prototypes should consider, in advance, electrical insulation, leakage current, and other safety indicators to avoid, during operation, influencing other equipment without being influenced by external interference. Rectification later is paid for with an astronomically high price, and that is a necessity to be faced in registration and testing.
Embedding the requirements of regulatory compliance into medical devices prototype development is actually a proactive risk management approach. This would avoid drastic design changes due to functionality issues arising during registration and testing or clinical validation-fundamentally saving much time and money-and acts as the cornerstone for speeding up product launch.
How Does ISO 13485 Certification Impact The Quality System For Prototype Development?
ISO 13485 Prototype elevates the process from a potentially haphazard activity to a controlled, traceable, and vital process that provides early confidence in the safety and effectiveness of the final product. It calls for a robust quality system right from the concept stage onward. This is particularly reflected in:
Strict Design Controls and Management of Documentation
For prototyping, it is required to have documentation of design input, output, review, and change according to the ISO 13485 standard. Fully documented specifications are required, from customer requirement specifications to drawing revision records, and design review conclusions. This also provides consistency between the prototype and the design intent and forms a useful "design history," providing direct evidence for subsequent registration applications.
Controlled Manufacturing and Traceability
This standard requires verification and control for key prototyping processes. For instance, in terms of the medical-grade materials involved, supplier qualification certificates and incoming inspection records should be provided; it also involves setup and confirmation of the parameters of processing equipment, such as layer thickness and laser power in 3D printing. This would ensure consistency and repeatability in this process of prototyping and give a clear base for future replication or iteration.
Early integration of risk management
Risk management activities should start early enough during prototyping to identify potential risks associated with design, materials, and processes for which control measures should be implemented. In that respect, it forces the team to think about requirements linked to safety in advance, such as cleaning and sterilization and biocompatibility, integrate the solutions into the design and manufacturing of the prototype, reducing the potential for major design changes later.
The value coming from a quality system throughout the entire prototyping process goes far beyond just obtaining a certificate in ISO 13485. This will produce, for the prototype itself, a standardized and transparent framework; not only a functional "sample" but a reliable, data-complete, and risk-controlled "pre-product".
Selection Of Medical-Grade Materials That Meet Biocompatibility Requirements?
Selection is the first hurdle in the determination of the product's biocompatibility and safety in the development of the medical device prototype. Selection of an appropriate material minimizes risks in registration but most importantly ensures patient safety. To make clear the selection logics, the key considerations are compared below.
| Dimensions of Consideration | Basic Path: Selection of Certified Materials |
Advanced Path: Biological Evaluation of Materials |
| Principle | The use of medical grade materials with mature certification comes first, such as USP Class VI or ISO 10993. | If an innovative material is used, a complete biological evaluation under ISO 10993 standards will be required. |
|
Applicable Scenarios |
Applies to most mature materials of low risk and having a clear, speedy route. | Applies to new, innovative materials or completely new human-human interaction scenarios of higher risk and cost. |
| Key evidence | Fully completed certification certificates and test reports from the material supplier. |
Commissioning a properly equipped and qualified laboratory to carry out the full or partial set of required safety tests. |
| Our Support | We provide fast matching and recommendations based on a validated database of medical grade materials, along with consultation on evaluation schemes and testing support. | We support clients in mitigating compliance risks along the entire process. |
We strongly recommend planning for biocompatibility right from the early stages of prototyping, selecting pre-certified materials from validated databases to minimize uncertainty. Where innovative needs are to be covered, the full biological evaluation path will be planned long in advance. This would avoid delays in registration and disruption in the designs owing to material issues right from the beginning and lay the foundation for the safety and rapid market launch of products.

Figure 2: Accurate volumetric control for medical prototypes by LS Manufacturing
What Standards Should Prototypes At Different Clinical Stages Meet?
Most people misinterpret medical device prototyping as an activity in which one version of a prototype tries to meet all needs. This often results in the waste of resources in usage or lack of standards. What science precisely supports at each step-from concept to clinical validation - is the making of "just right" prototypes, based on the goals of different clinical stages.
| Clinical Stage | Prototype Core Goals | Production Standards and Strategies |
| Proof of concept | Verify the feasibility of the design concept as soon as possible. | Economical and rapid methods, like 3D printing, can achieve core functions. Materials are limitless; now is the time for rapid iteration. |
| Functional Testing |
Conduct comprehensive engineering testing of prototypes. |
Thorough validation of product performance and reliability using prototypes that are close representations of product size, materials, and performance. |
| Animal testing | The first step in the procedure is to assess the safety of the product in question and its biocompatibility. | It has to be prepared from biocompatible medical-grade material following aseptic processing so that the tests are valid. |
| Clinical Validation | Safety and efficacy data should be obtained in trials conducted in humans. | The prototype must be representative of the final product and should conform to medical device norms with respect to performance, materials, and manufacturing processes. |
Generally speaking, medical device prototyping works effectively with a dynamic but progressive approach. From the "economical" proof-of-concept stage up to the "quasi-commercialization" stage, with clinical validation, each stage of prototype design should set certain goals with matching standards. Such a strategy will effectively keep early-stage R&D costs in check while making sure the integrity and reliability of the prototype data chain are ensured, thereby evading project delays or failures because of mismatched prototype standards.
How Can Precision And Manufacturability Be Balanced In The Prototype Of Surgical Instruments?
In the process of surgical instruments prototype development, the aim is to not only achieve better functional precision but also prove that its design can be stably and economically transformed into mass-produced qualified products. The principles of design for manufacturability should be given priority attention. Therefore, the following points must be taken into consideration:
Structural Simplification and Assembly Optimization
While ensuring functionality, the number of parts should be minimized and complex internal structures avoided. For example, integrated 3D printing technology could be used in place of traditional multi-part assembly structures or self-locking snap-fit designs could be adopted instead of screw fastening. In so doing, later assembly difficulty and time can be greatly reduced, hence improving production efficiency and consistency.
Matching Manufacturing Processes and Tolerancing Design
In the stage of prototyping, the processes involved in mass production should be considered, such as precision injection molding and 5-axis machining, and reasonable tolerances should be set. It is not necessary to pursue extreme precision if the mating dimensions are not critical; otherwise, the cost for processing will increase sharply. Designers need to understand the capability boundaries of different processes and leave reasonable margins for manufacturing errors while meeting clinical requirements.
Feasibility of mass production of materials and surface treatments
Material selection in the case of a prototype should meet not only the requirements of biocompatibility and mechanical properties but also consider supply chain stability and cost in mass production. Meanwhile, special surface treatments, such as antibacterial coating, should be able to be stably and uniformly implemented on batches of parts rather than laboratory samples.
A successful prototype of a surgical instrument incorporates manufacturability considerations right from the beginning. It identifies and optimizes possible manufacturing, quality, and cost problems for large-scale production. The idea is to avoid designing a "perfect" product that is either unproducible or too expensive to make, and also to ensure that transition from prototype to mass production is smooth.
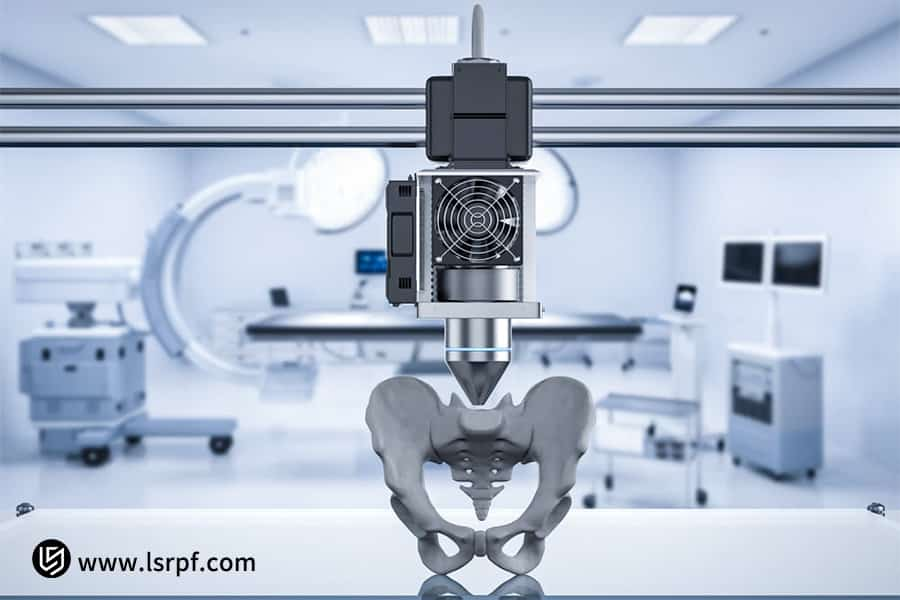
Figure 3: Accelerated development of bespoke surgical implants by LS Manufacturing
How Does Rapid Prototyping Technology Facilitate Innovation In Medical Devices?
The most important value of rapid prototyping for medical devices is that it can shorten the time period from conceptual design to physical verification, providing a very effective platform for trial and error and iteration. Among these technologies, medical grade 3D printing is playing a revolutionary role, with its advantages and requirements particularly reflected in:
- Achieving Complex Structures and Personalized Customization: Complex microchannels and porous trabecular bone structures, difficult to process by traditional methods, can easily be produced with medical grade 3D printing. Personalized surgical guides and implants, therefore, can be manufactured to exactly conform to the anatomy of the patient, a development that directly contributes to precision medicine.
- Integration of several materials and functions: These modern rapid prototyping technologies enable components to be manufactured from a range of materials that vary between rigid and flexible to biodegradable, all within one component. For example, an integrated component combining a rigid framework with a soft seal can be printed in one step for use in the anatomical models that simulate real organs or prototypes of complex drug delivery devices.
- Meeting particular requirements of medical applications: Safety and compliance can never be sacrificed in rapid prototyping for medical devices, no matter how much speed is at stake. Materials must be biocompatible, and the printing process itself has to be validated in such a way that it ensures consistency from batch to batch and traceability throughout the process according to quality system requirements.
These are a class of rapid prototyping technologies wherein medical grade 3D printing enables the R&D team to explore previously impossible designs at a lower cost and in record time. The rigorous control over materials and process ensures that innovation follows the compliant track right from the initial stage up to clinical validation and product registration.
How Does LS Manufacturing Help Minimally Invasive Surgical Instruments Get Over The Technological Bottleneck?
The core value in the R&D of innovative medical devices that we offer relates to its medical device prototyping services in helping clients overcome the key technological bottlenecks in launching their products. The following is a typical case illustration that demonstrates the application of the LS Manufacturing medical prototype approach:
Client Challenge
In the process of developing a new kind of minimally invasive surgical instrument - an intracavitary anastomosis device-an innovative company came up with huge obstacles during animal tests. The core transmission mechanism demonstrated significant lag in operation and could not provide surgeons with effective and precise control of the anastomosis action. Restricted by the traditional manufacturing process, there was no more room for further optimization due to its complicated inner transmission structure.
LS Manufacturing Solution
Intervening, our engineering team immediately abandoned the traditional machining method. We employed medical grade stainless steel 3D printing technology, manufacturing a one-step and one-piece transmission system with a superior mechanical path and extreme minimal clearance. This did not only solve the lag problem; further improvement had electropolishing applied on this complex one-piece molded cavity, giving it a surface finish far exceeding that of standards and perfectly meeting the extremely high requirements of sterility and cleanliness for surgical tools.
Results and Value
In subsequent animal trials, the newly manufactured prototype worked perfectly, and the operational response speed and accuracy of the instrument improved by about 60%, winning high praise from clinical experts. This technological breakthrough thus directly advanced the overall project schedule of the client by at least 5 months and saved over 2 million RMB in R&D costs incurred from repeated trial and error and mold modification.
We applied advanced technologies, such as medical grade 3D printing, to overcome common structure, material, and performance limitations of minimally invasive surgical instruments for our clients. What sounded like impossible technical challenges became the key product competitiveness, securing valuable time-to-market windows and huge cost advantages for our clients.

Figure 4: Precision dosing in medical prototype fabrication by LS Manufacturing
What Type Of Technical Documentation Does Prototype Development In Medical Devices Require?
Full technical documentation is the cornerstone of successful medical device registration. It serves mainly to comprehensively document the process of design control and to provide evidence on the safety and effectiveness of the product. This will not only be a roadmap for internal development but will also serve as evidence to the regulatory agencies of the enterprise systematic and compliant development capability. It shall include, but shall not be limited to, the following key documents:
- Design Input and Output Documents: This is the beginning of the documentation system: design inputs, which shall record user needs, clinical functions, performance indicators, and regulatory standards in a clear manner. Design outputs will include product drawings, technical specifications, parts lists, etc. Every input should be verified to have a corresponding output.
- Material Certification and Biocompatibility Documentation: Supplier certifications and material certificates of all medical-grade materials, taken together with biocompatibility assessment or test reports as relevant to parts of USP Class VI or ISO 10993, should be available. This directly relates to establishing the product's biocompatibility.
- Manufacturing Process Validation Records: Demonstrate that the prototype manufacturing process is consistent and controllable for each critical process involved in its creation, such as 3D printing parameters or sterilization processes, via detailed process parameter records, equipment calibration certificates, and first-article inspection reports.
In other words, it means the creation of technical documentation as a basic activity that runs parallel to prototyping prepares for efficient project progress; it is not an afterthought. A good design control documentation system, such as the "birth certificate" of the product, clearly shows the design trajectory from concept into prototype.
How To Plan The Registration Application Path During The Prototyping Stage?
Based on the sound preparation work of registration application, accelerate medical device development can be assured, and in this process, every piece of data generated is to become strong support for future registration documents. That is to say, the following three levels should be systematically planned:
Clearly Defining Requirements Regarding Evidence of Registration
The prototype design input phase demands the in-depth analysis of regulatory requirements of the target market. Main headings that have to be checked during the review of registrations, such as performance indicators, biocompatibility, and electrical safety, are translated into specific technical parameters to be verified by the prototype. That would ensure the development of a prototype on the right compliant track from the beginning.
Transform the prototype validation data into registration evidence
The prototype shall undergo functional, lifespan, and usability testing that complies with the requirements of the quality system. All tests must be done with a pre-approved standard protocol, which should include a complete record of raw data and a standardized report. This will be highly controlled data that may then be directly submitted as core evidence for future design validation, thus avoiding repeat testing.
Establish a transparent system for design history documentation
It should provide a complete record of the process flowing from design input to review, changes, and output. This naturally formed documentation system in the design control process can prove to be the best evidence to prove to regulatory agencies that the design process is rigorous and controlled, which can greatly improve the efficiency and success rate of registration reviews.
In other words, planning registration application integration at the prototype phase is the best lever to accelerate medical device development, in that it strongly cuts down on high costs and time delays incurred in later phases of the project because of supplementary registration data, while improving the standardization and success rate of the whole R&D process from the very beginning, laying a solid foundation for rapid and stable market launching of products
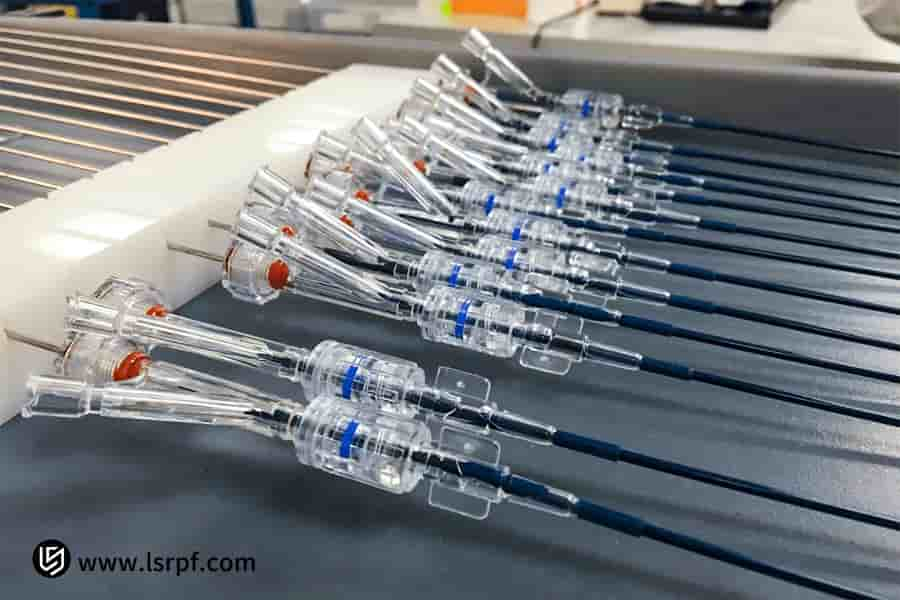
Figure 5: 360-degree prototype for functional validation by LS Manufacturing
FAQs
1. What specific requirements must be followed during the development of medical prototype devices?
Furthermore, beyond the basic functionality, one needs to proactively address meeting the particular standards for medical devices. We rigorously apply the quality system of ISO 13485 to ensure that these prototypes meet regulatory requirements in biocompatibility, sterilization tolerance, electrical safety, and other aspects at the very beginning of their registration.
2. How long does it take from concept to getting the first functional prototype?
It depends on the complexity of the device. Relatively simple devices take about 2-3 weeks, while more complex ones with precision transmissions and electronic components take 4-6 weeks. We have an expedited channel to flexibly address urgent R&D needs and fully guarantee your project schedule.
3. Is it possible to make small batches of clinical trial devices?
Of course, one of our various services is small batches of clinical trial device fabrication, ranging from 10-100 units. Manufacturing was by medical device manufacturing standards, and each unit will be fabricated to the demanding performance, quality, and consistency for clinical trials.
4. How do you ensure the biocompatibility of prototype materials?
We control risks from the source through the strict selection of medical-grade materials with authoritative certifications such as ISO 10993 or USP Class VI. Complete material certification documents can be provided together with the prototype, providing substantial evidence for your biocompatibility assessment.
5. How do you protect our IP during the prototyping process?
Information security is our lifeline. Every project starts with a legally binding confidentiality agreement, and internally, we apply a strict data hierarchy management and access control system. If requested by the client, physical and information firewalls may also be established.
6. What type of medical device prototypes do you support?
The services offered by our company range from active devices, such as diagnostic equipment, to passive ones, including surgical instruments, implants, and non-implantable devices. LS Manufacturing is well-versed with regard to the regulations and processes associated with various products.
7. How to make the prototype data available for use in applications for subsequent registration?
Apart from prototypes, we provide a complete quality evidence package, which includes detailed production records, process inspection reports, and performance test data. All are generated to specifications and can be directly usable as design verification material to support applications for registration.
8. How to start a medical device prototype project?
The process for getting started is pretty simple: just fill in some initial concept sketches or technical requirements, and we will make sure that a senior engineer connects with you within one business day to discuss the technical solutions in depth and provide you with an initial project assessment and quotation.
Summary
Medical device prototypes are much more than a technical realization; they are the cornerstone of risk management, regulatory compliance, and commercial success. Finding the right prototyping partner lays a sound foundation for the whole product life cycle.
Therefore, if you want to speed up R&D while reducing registration risks, please contact our experts in medical devices today for a "Registration-Oriented Prototyping Solution" for your project! Let us apply our professional experience in the manufacturing of medical devices to protect your journey of innovation.
📞Phone: +86 185 6675 9667
📧Email: info@longshengmfg.com
🌐Website: https://lsrpf.com/
Disclaimer
The content on this page is for informational purposes only. LS Manufacturing makes no representations or warranties, express or implied, regarding the accuracy, completeness, or validity of the information. It should not be inferred that third-party suppliers or manufacturers will provide performance parameters, geometric tolerances, specific design characteristics, material quality and type, or processes through the LS Manufacturing network. The buyer is solely responsible for this information. For parts quotations, please specify the exact requirements for these parts. Please contact us for more information .
LS Manufacturing Team
LS Manufacturing is an industry-leading company specializing in customized manufacturing solutions. With over 20 years of experience serving more than 5,000 clients, we focus on high-precision CNC machining , sheet metal fabrication , 3D printing , injection molding , metal stamping , and other one-stop manufacturing services.
Our factory boasts over 100 state-of-the-art five-axis machining centers and is ISO 9001:2015 certified. We provide fast, efficient, and high-quality manufacturing solutions to customers in over 150 countries and regions worldwide. Whether it's small-batch production or mass customization, we can meet your needs within 24 hours. Choosing LS Manufacturing means choosing efficiency, quality, and professionalism.
For more information, please visit our website: www.lsrpf.com .