Most medical devices face numerous challenges during the innovation process, including lengthy R&D cycles, high development costs, and complex regulatory requirements. Many promising solutions fail due to inefficiencies and slow iterations of traditional methods, missing valuable market opportunities.
Today, rapidly and accurately translating ideas into high-quality prototypes for rigorous testing and regulatory approval has become a critical factor in determining the success or failure of innovation. LS Manufacturing, by integrating advanced manufacturing processes, materials science knowledge, and medical device quality systems, builds a "high-speed highway" from concept to product for its clients, effectively shortening time-to-market by more than 40% and significantly controlling development costs.
Figure 1: Medical device rapid prototyping and innovation solutions by LS Manufacturing
Medical Device Rapid Prototyping Quick Reference Table
| Core Dimensions |
Key Content Description |
| Primary Objectives |
Accelerate design iteration, reduce development costs and risks, and provide high-quality physical evidence for clinical testing and regulatory approval. |
| Key Technologies | Includes a wide range of cutting-edge technologies such as 3D printing (SLA, SLS, FDM), silicone molding, rapid CNC machining, and vacuum injection molding. |
| Material Selection | Medical-grade photosensitive resin, silicone, ABS, PC, PEEK, PMMA, etc., are provided to simulate the mechanical and biocompatibility of the final product. |
| Key Values | Greatly reduces the R&D cycle by up to 40%+, allows early functionality, assembly, and ergonomic testing, and helps in keeping total project costs under control. |
| Quality Considerations | It should rigidly adhere to design inputs, emphasizing dimensional accuracy, surface quality, mechanical strength, and compatibility with the sterilization methods. |
| Regulatory Support | Prototypes can be used in creating Design History Files, supporting feasibility studies, and providing preliminary data for formal registration. |
| Applicable Stages | Applies throughout the entire productization process, from proof-of-concept and detailed design to design conversion before design freeze (hand prototype). |
Systematic rapid prototyping is not just model making anymore; it is a strategic element deeply integrated into the medical device development process. By rapidly realizing physical prototypes, it provides critical evidence toward design decisions that effectively bridge the gap between creative and compliant products, acting as an accelerator for driving innovative success to market.
Why Trust This Guide? Practical Experience From LS Manufacturing Experts
The reliability of this guide is based on the systematized expertise developed by LS Manufacturing in rapid prototyping services for medical devices. Our engineering team, specializing in the design, development, and production of medical devices, has years of experience working with the world's top-ranked medical device companies, thus allowing us to provide insightful recommendations from manufacturability, testability, and final compliance standpoints.
This systematic capability, deeply integrating engineering knowledge with medical regulations, ensures that we provide not just prototypes, but key foundational steps toward successful mass production.
LS Manufacturing has helped customers complete multiple functional iterations of the most complicated surgical instruments within two weeks, which then passed animal trials successfully. We have created prototypes for Active Device clients, meeting the demands of precision structure, waterproof sealing, and even biocompatibility testing to quickly move into clinical approval.
These success stories across orthopedics, dental, cardiovascular, and diagnostic devices have given us a deep understanding of different products' special needs and possible risks at the prototyping stage.Therefore, it is not only technology that we share but also invaluable experiences distilled from countless practical cases—experiences that can effectively avoid pitfalls and improve efficiency.
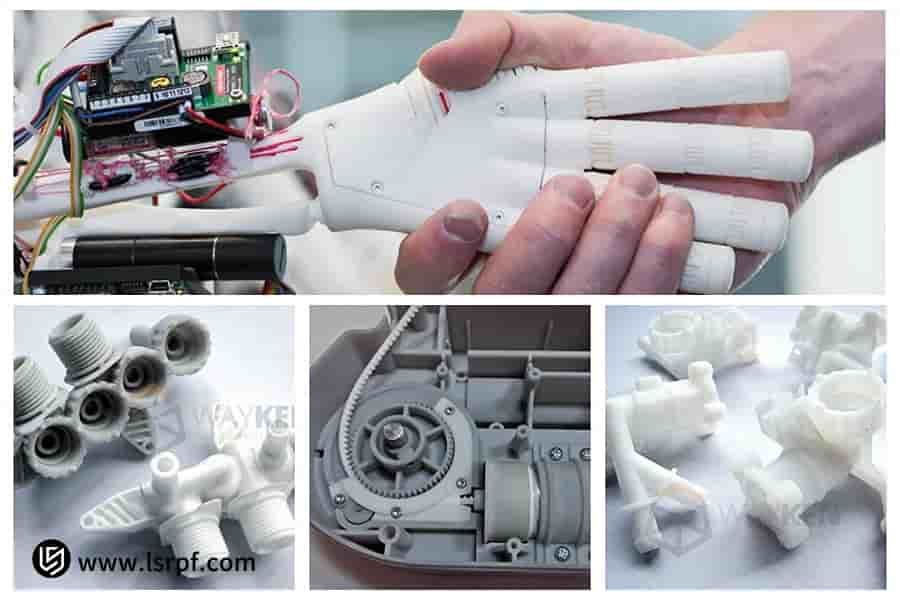
Figure 2: Cost-effective medical device prototype with FDA compliance standards
How To Save From High Modification Cost Later By Early Design Verification?
Within the medical device development process, there is a widely accepted "tenfold rule": the cost of discovering and correcting a problem at the design step can increase tenfold at the prototype construction phase. If the problem continues through into mass production or even to clinical trials, correction costs will increase exponentially.
Therefore, the most cost-effective way of controlling the overall risk is thorough validation in the early design stage. Key validations can be achieved before expensive molds and production costs are invested in through systematic rapid prototyping of medical devices:
1. Structural and Functional Validation:
By using cost-effective medical prototypes, teams are quickly able to get physical prototypes for real assembly, motion testing, and basic functional demonstrations of their idea. This effectively exposes design flaws, interferences, or inappropriate material selection, thus avoiding significant later modifications or even mold scrapping losses.
2. Ergonomics and User Experience Feedback:
What looks perfect on screen may not turn out to be the most user-friendly design once in operation. Touch-enabled, operable high-fidelity prototypes allow the end-users, doctors and nurses, to provide early feedback that will optimize a product's human-computer interface, which then leads to enhanced market acceptance and clinical value.
In that respect, the investment of resources in rapid prototyping and design validation at the early stages is a very cost-effective risk management approach. Finding and fixing problems at an early stage fundamentally avoids huge modification costs and time delays that may happen later on, thus it is worth the investment to ensure smooth project progress and successful market launch.
How Does Multi-Material Prototyping Accelerate Product Functional Testing?
A large portion of products in medical device development rely heavily on the functionality of combinations of . Traditional single-material prototypes often fail to simulate real-world conditions, while advanced technologies like medical-grade 3D printing and rapid prototyping enable the manufacturing of functional test prototypes with different properties, such as flexible and rigid components, transparent, or non-transparent materials that revolutionize early-stage validation.
1. Real-life simulation generates reliable data:
Through the use of multi-material integration technology, functional prototypes featuring complex structures like soft sealing rings, rigid shells, or flexible hinges can be created. As such, engineers can perform near-realistic simulation tests in the lab environment, such as the compliance of catheters and opening and closing sealing performance of valves, thereby obtaining valuable early functional test data and significantly reducing reliance on later pilot production.
2. Precise Material Selection, Minimizing Clinical Risks:
LS Manufacturing produces a wide variety of biocompatible and medical-grade 3D printing materials. Using safe materials, customers can make prototypes early in development to test not only mechanical functions but also perform initial chemical compatibility and sterilization tolerance assessments. This advanced material selection and validation can effectively identify potential risks, thus laying a solid foundation for product biosafety and shortening subsequent compliance approval cycles.
Multi-material prototyping significantly enhances functional testing. Iterative optimization using high-fidelity physical prototypes shortens the "design-test-modify" cycle significantly, hence accelerating the overall product maturation process and reducing the cost and risk of major changes due to material or design defects later on.
What Are The Important Considerations For Prototypes That Meet FDA Submission Requirements?
The creation of prototypes for FDA submission involves much more than the attainment of form and function; it is actually all about meeting very strict regulatory requirements because the prototype itself has to serve as reliable, traceable scientific evidence that supports claims of design safety and effectiveness. This necessitates that the whole manufacturing process be done within a robust quality system framework.
1. Materials and Process Compliance:
The selected materials should have clear biocompatibility certifications, such as USP Class VI, and their processing methods, such as medical-grade 3D printing, should be validated to make sure that the mechanical properties, dimensional accuracy, and batch-to-batch consistency of the prototype meet established standards and satisfy the basic prerequisite for data reliability that the regulatory requirements call for.
2. Full-process traceability:
From design documents, material batch numbers, processing parameters to post-processing records, the full-process traceability of the entire manufacturing chain should be realized. The quality system constitutes the foundation in guaranteeing that all prototypes are traceable and can clearly reproduce the manufacturing process in application and respond to inquiries set forth by review agencies.
3. Comprehensive Technical Documentation Support:
LS Manufacturing not only provides physical prototypes, but also packages them with a set of technical documentation, including material certification certificates, process validation reports, and testing data. This can be seamlessly accommodated into your design history files, constituting very strong evidence of the validity of the prototype presented to the FDA.
Essentially, FDA-compliant prototype manufacturing advances the concept of production quality management to the very start of the R&D process.
By using standardized processes to ensure conformance with a quality system, authenticity of prototype data, traceability, and reliability of results, we can support with efficiency the demanding regulatory requirements of the application process and drastically reduce the risk of review delays caused by incomplete documentation.

Figure 3: Accelerated medical prototyping process for faster time-to-market
How Does The ISO 13485 System Ensure The Quality Consistency Of Prototype Production?
In medical device R&D, prototypes are the core basis for design verification. The production of ISO 13485 prototypes is not merely model fabrication; rather, it falls within an internationally recognized quality management system framework. Its fundamental goal is to ensure consistent quality from the first prototype through to the final batch, providing reliable data for regulatory applications and production transformation. This goal will be achieved through continuous process control.
| Core Elements of the Quality System | Their Particular Implementation and Assurance Role in Prototype Manufacturing |
| Design Control and Document Management | Standardize design input and change control processes to make sure that prototypes actually reflect design intent and avoid version errors. |
| Process control and verification | Standardization of materials, equipment, and processes to ensure consistency and repeatability of prototype performance within different batches. |
| Record Keeping and Traceability | Full records are kept of the batch number of materials and process parameters to provide full traceability from start to finish, thereby supporting data reliability and deviation investigation. |
Integrating the ISO 13485 system into prototyping systematizes manufacturing activities through standardized process control. This ensures consistent quality and makes the prototype data reliable evidence to support clinical trial applications and mass production transformation.
What Is The Rapid Production Strategy For Small-Batch Clinical Trial Samples?
Clinical research is an important stage for medical devices to verify their safety and performance. The demand for clinical samples has distinct characteristics: moderate quantity, usually tens to hundreds of pieces; high consistency in quality with the final product; and extreme sensitivity to time. Traditional mass production molds are costly and take a lot of time. Therefore, innovative small-batch production strategies become the best solution for rapid delivery in such situations.
1. Using rapid mold making and hybrid manufacturing technology:
Unlike the time-consuming and high-cost, all-steel mass production mold, the rapid molds are made of such materials as aluminum alloys or mild steel, combined with efficient injection molding processes. This solution can deliver hundreds of high-quality clinical samples within weeks, with physical properties and materials highly consistent with the final product, at a fraction of the cost of traditional mold making. This is a core means of achieving rapid delivery and cost-effectiveness.
2. Process Stability Guaranteed by a Quality System:
The whole manufacturing process, from material certification and process parameter verification to end-to-end inspection, is under the stringent ISO 13485 quality system to make sure each batch of clinical samples shows excellent quality consistency. Complete technical documentation provided can be directly used to support clinical trial applications, providing reliable process data for subsequent large-scale production.
By integrating cutting-edge processes like rapid prototyping into a robust quality system, we can offer customers a "high-speed pathway" for producing small batches of medical devices. This will precisely match the comprehensive needs of speed, quality, and cost in the clinical stage by guaranteeing speedy delivery of quality clinical samples, hence greatly shortening the entire process of clinical research and product launch.
How Does LS Manufacturing Cut 6 Months From Surgical Instrument Time-To-Market?
Design validation is crucial for minimally invasive surgical instruments because of complex mechanical structures with stringent performance requirements. Fast iteration will be very important to shorten the time-to-market significantly. The following case study shows how LS Manufacturing helps its clients gain a market advantage through technology integration.
1. Client Challenge:
A medical instrument company's innovative laparoscopic surgical forceps underwent multiple critical structural modifications during development to optimize the jaw closure force and transmission efficiency. With each design validation cycle traditionally outsourced for machining, it would take 4-6 weeks, which slowed things down and made the unit cost so high that the team did not dare to iterate further, stalling the project.
2. LS Manufacturing Solution:
We quickly assembled an engineering team and followed a combined strategy for different validation objectives: the core plastic structure was realized via high-precision medical-grade 3D printing, with SLA obtaining samples in 24-48 hours; metal 3D printing was used for critical metal transmission components to achieve one week of iteration. Upon freezing the design, rapid prototyping of aluminum molds was immediately started with simultaneous implementation for small-batch trial production of functional prototypes, and the verification cycle for each modification was consistently reduced within one week.
3. Results and Value:
With an efficient rapid iteration process, the total development cycle of the product was successfully shortened from the expected 18 months to 12 months, with a decrease in overall development cost of 35%. More importantly, high-quality prototypes and trial production samples ensured smooth clinical validation, helping the product to be launched six months in advance, securing a valuable market window.
This case demonstrates that systematically applying advanced, medical-grade 3D printing and rapid prototyping technologies to the development process can directly translate iteration efficiency into time and market advantages, providing an effective path for high-tech medical devices to achieve their goal of shortening time to market.
How To Further Optimize Prototype Manufacturing Costs Through Supply Chain Integration?
In the manufacturing of medical device prototypes, explicit processing costs are only a portion of the cost; implicit costs regarding material management, multiple iterations, and time delays are just as important. For cost-effective medical prototypes, systematic supply chain optimization and refined strategies for cost control become necessary. The following are ways in which LS Manufacturing optimizes costs:
1. Vertical Integration and Centralized Procurement:
By establishing a vertically integrated production system and a large-scale centralized procurement network, the procurement cost of raw materials and expenses of external collaboration could be effectively reduced. This integration cuts out all the middlemen markups, making the medical-grade material more accessible to customers at better prices and thus laying the foundation for cost-effective medical prototyping right from the source.
2. Internal Process Collaboration and Efficiency Improvement:
We centralize the management of the design, 3D printing, finishing, and quality inspection processes to avoid communication costs and time losses brought about by cross-plant collaboration. Smooth internal integration of the processes reduces wait times in production, increases iteration speed, and effectively controls costs by enhancing efficiency across the board.
3. Lean Logistics and Inventory Management:
We implement a lean logistics strategy that greatly reduces logistics and warehousing costs per prototype through standardized packaging, bulk shipping, and optimized inventory levels. This refined operational management is another important part of supply chain optimization and further helps customers economize on overall expenses.
Through end-to-end supply chain optimization, from materials and production to logistics, LS Manufacturing makes hidden costs explicit and controls them effectively, which allows us to consistently keep delivering truly cost-effective medical prototyping solutions for our customers and to translate the cost advantage into an innovative competitive advantage.

Figure 4: Custom medical components prototyping with ISO 13485 quality system
What Are Essential Factors To Be Considered For Smooth Transition From Prototype To Mass Production?
The leap from prototype to mass production is the most difficult step in the course of developing medical devices, and quite a lot of innovative products are stuck at this stage simply because they cannot achieve a successful transition into mass production.
The key to a successful transition is preparing for that transition from the very beginning of prototype development, introducing design for manufacturability principles to ensure the prototype not only meets functional testing requirements but is also able to support large-scale production. The following factors should be emphasized to achieve a seamless transition:
1. Early Integration of Design for Manufacturability (DFM) Analysis:
During the design of the prototype, LS Manufacturing engineers make early interventions to conduct DFM analysis from the perspective of material selection, structural processes, and assembly procedures. For instance, analyzing for flaws such as the existence of undercuts that cannot be demolded, uniform wall thickness, and whether standard parts could be applied, optimizing the design from the very beginning and avoiding potential problems for the subsequent small-batch medical device production and large-scale mass production.
2. Employ prototyping technologies compatible with mass production processes:
After initial validation, focus on technologies that are consistent with or very similar to the final mass production process, such as injection molding, for high-fidelity prototypes or small-batch production of medical devices. For instance, rapid prototyping to manufacture pilot-production samples enables early validation of mold design, identification of potential molding defects, and acquisition of real-world process parameters, building up reliable data for transition into mass production.
3. Establish consistent quality and data throughout the process:
The whole process, from prototyping to mass production, should be done in accordance with the same quality system, for example, ISO 13485. Material testing data, process parameters, and validation results obtained at the stage of prototyping should be used as a basis for validation of mass production processes with the aim of ensuring consistent product performance and data traceability that will guarantee easy transition to mass production.
Successful transition in mass production does not happen overnight but is dependent on early systematic manufacturability design planning, a prototyping strategy aligned with mass production processes, and consistent quality management. This proactive strategy minimizes the industrialization risks and greatly shortens the product launch cycle.
How To Choose A Prototyping Partner With Medical Device Expertise?
The choice of medical device prototyping partner will directly affect their contribution to product development efficiency, cost, and, finally, success. A really professional partner has to be deeply aware of the special requirements for the medical device industry, beyond mere model fabrication. We recommend evaluating them on the following key dimensions:
1. Authoritative Quality Certification as the Basic Threshold:
First of all, it is necessary to verify whether the service provider is certified to ISO 13485 Medical Device Quality Management System certification. It's not just a certificate; it means that the partner has implemented a standardized and documented process that ensures quality, traceability, and integrity of data throughout the prototyping process, which is so important for subsequent regulatory filings.
2. Deep Industry Experience as a Core Value:
Analyze if they have extensive industry experience in medical device prototyping projects, especially successful cases in areas similar to your product, such as cardiovascular, orthopedic, minimally invasive surgery, etc. Extensive experience will enable them to foresee common pitfalls in development. They are of great value to provide insight into manufacturability and testability from an early stage of product design, thus avoiding detours that aren't necessary.
3. Full-service technical capabilities and collaborative service model:
A good partner will have a broad portfolio of technologies, from medical-grade 3D printing to rapid prototyping, and will be able to recommend the most cost-effective and efficient solutions based on your needs through the various stages, including validation and small-batch pilot production. More importantly, their service model should be deeply collaborative, embedding themselves in your development process as an engineering support team, providing end-to-end support from design review to production transformation.
A professional partner, in an ideal world, should be your R&D extension. LS Manufacturing builds professional barriers with comprehensive quality certification and cross-industry experience, guaranteeing smooth transition from prototype to mass production through an "end-oriented" collaborative service model to successfully accomplish your project.
Figure 5: Medical-grade rapid prototyping for surgical instrument development
FAQs
1. How long does it take to prototype a medical device?
This depends on product complexity and process selection. Normally, for a typical project, from receiving manufacturable data to delivery of a fully functional prototype, it takes 1-4 weeks. We also have a fast-response channel for urgent needs; through optimized scheduling and concurrent engineering, we can fully guarantee your project schedule.
2. What kind of materials do you use for medical prototyping?
We provide a wide library of biocompatible materials, including dozens of medical-grade photosensitive resins, engineering plastics such as ABS, PC, and PEI, and metals including titanium alloys and stainless steel, all USP Class VI-certified. These accurately simulate the mechanical, chemical, and biological properties of the final product.
3. How does prototyping support FDA 510(k) filing?
The key is fully traceable technical documentation. We are thorough in following our quality system for providing a complete documentation package: material certification certificates, records of process parameters, and test reports. This constitutes important objective evidence supporting the design validation and verification requirements of the 510(k) filing.
4. What's the MOQ for small batch production?
We are committed to offering a highly flexible service; hence, we thoroughly understand the needs of the clinical trial phase. Thus, we do not have a rigid MOQ; our acceptance of order can be from just a few pieces to several thousands of pieces to ensure maximum convenience and support to our clients.
5. How do you ensure that information regarding prototypes remains confidential?
We consider confidentiality to be the core of all our commitments. We enter into legally binding confidentiality agreements with our clients, besides establishing a general security management system for data access and storage through ISO 13485 certification. This best protects your intellectual property.
6. Do you offer prototype design optimization?
Yes, before production, our senior engineers will offer free manufacturability design analysis and give optimization suggestions from the perspective of manufacturability, cost control, and assembly to help you improve the rationality of your design and avoid subsequent risks.
7. Are the quality standards in samples from clinical trials identical to those in mass production?
Yes, the clinical samples are manufactured in the same quality system as the final product. Materials, processes, and test standards are exactly the same as the production lots to make sure that sample data is representative, effective for product performance evaluation, and clinical evaluation of the product.
8. How do I get a detailed quote and timeline for prototype production?
You are required to provide only the 3D model file of your product and the technical requirements. Our expert team starts the process analysis and presents a comprehensive solution with a detailed quotation, exact timelines, and professional advice within 24 hours.
Summary
Rapid prototyping in medical device development is no longer purely a technical task; rather, it has become one of the determining factors of both project efficiency and success. It builds one of the key competitive advantages through early verification of risks and accelerates design iteration. How to best make this successful leap from an idea into the market is by engaging a professional partner who has profound industry knowledge, paired with a strong quality system.
Time is opportunity, and efficiency determines market structure. Let us not allow brilliant ideas to be defeated because of inefficient prototyping. The professional team from LS Manufacturing is ready to support you in this process. Feel free to contact us today for a free, in-depth design feasibility analysis and a project quote tailored just for you.
Let us capitalize on our proven rapid prototyping solutions and wide-ranging project experience to clear the obstacles in your development path and jointly drive your innovative products to market more efficiently with less risk, giving you a competitive edge!
📞Tel: +86 185 6675 9667
📧Email: info@longshengmfg.com
🌐Website:https://lsrpf.com/
Disclaimer
The contents of this page are for informational purposes only. LS Manufacturing services There are no representations or warranties, express or implied, as to the accuracy, completeness or validity of the information. It should not be inferred that a third-party supplier or manufacturer will provide performance parameters, geometric tolerances, specific design characteristics, material quality and type or workmanship through the LS Manufacturing network. It's the buyer's responsibility. Require parts quotation Identify specific requirements for these sections.Please contact us for more information.
LS Manufacturing Team
LS Manufacturing is an industry-leading company. Focus on custom manufacturing solutions. We have over 20 years of experience with over 5,000 customers, and we focus on high precision CNC machining, Sheet metal manufacturing, 3D printing, Injection molding. Metal stamping,and other one-stop manufacturing services.
Our factory is equipped with over 100 state-of-the-art 5-axis machining centers, ISO 9001:2015 certified. We provide fast, efficient and high-quality manufacturing solutions to customers in more than 150 countries around the world. Whether it is small volume production or large-scale customization, we can meet your needs with the fastest delivery within 24 hours. choose LS Manufacturing. This means selection efficiency, quality and professionalism.
To learn more, visit our website:www.lsrpf.com.